November 8, 2017
Explainer: hydrofluorocarbons saved the ozone layer, so why are we banning them?
On October 28, Australia ratified the Kigali Amendment to the Montreal Protocol.
Jenny Fisher, University of Wollongong and Stephen Wilson, University of Wollongong
Australia is the tenth country to ratify, joining others as diverse as Mali, Norway and Rwanda in a global commitment to dramatically reduce hydrofluorocarbons (HFCs) in the atmosphere. Once 20 countries have ratified the amendment, it will become binding.
HFCs were designed specifically to replace ozone-destroying compounds previously used in air conditioners and refrigerants. Unfortunately, we now know that HFCs are massively potent greenhouse gases – thousands of times more powerful than carbon dioxide (albeit released in far smaller quantities).
Read more: The 30-year-old ozone layer treaty has a new role: fighting climate change
If the Kigali Amendment becomes binding, the hunt will begin for a replacement for HFCs and their uses in industry. In a strange twist, the least environmentally harmful option may well be carbon dioxide.
Where do HFCs come from?
HFCs are made of carbon, fluorine and hydrogen. They are exclusively synthetic, meaning they have no known natural sources. To understand why they came into existence requires a quick history lesson.
Throughout the second half of the 20th century, another class of compounds called chlorofluorocarbons (CFCs) were widely used. CFCs are very stable, which made them ideal for many practical uses, including in refrigeration, foam packaging, and even aerosol cans for hair spray.
However, scientists soon discovered that CFCs had a major downside. Because they are so stable, they can survive in the atmosphere long enough to eventually reach the ozone layer. Once there, they break down in sunlight and destroy ozone in the process.
Read more: Explainer: what is the Antarctic ozone hole and how is it made?
The Montreal Protocol was a global agreement developed to stop this harmful ozone destruction. The protocol mandated a time frame to completely abolish CFCs. To replace them, new compounds were developed that do not destroy ozone: HFCs.
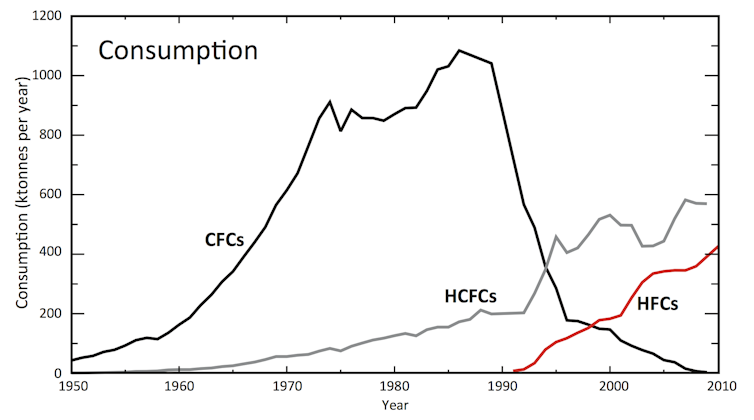
But the solution to one environmental problem became the cause of another: these replacements are potent contributors to warming the climate.
Why are HFCs so bad?
All greenhouse gases work by absorbing infrared radiation, which would otherwise escape into space. But not all greenhouse gases are created equal. The potency of a greenhouse gas depends on three properties:
-
how long it remains in the atmosphere (its “lifetime”)
-
how much radiation it absorbs
-
whether the specific wavelength of radiation it absorbs would otherwise be absorbed by something else in the atmosphere (like water).
Combined, these three properties can be used to determine the global warming potential for each greenhouse gas. This is a measure of how potent the gas is relative to carbon dioxide (CO₂). By definition, CO₂ has a global warming potential of 1. Methane, commonly considered the second most important greenhouse gas, has a global warming potential of 34 – meaning that 1 tonne of methane would trap 34 times more heat than 1 tonne of CO₂.
The global warming potentials for the three most abundant HFCs range from 1,370 to 4,180. In other words, these gases trap thousands of times more heat in our atmosphere than an equivalent amount of CO₂.
What will replace HFCs?
The nearly 200 countries that signed the original Montreal Protocol have unanimously agreed that the climate risks posed by HFCs are too significant to ignore. Developed countries will begin phasing out HFCs in 2019. Developing countries will follow suit between 2024 and 2028.
So what will our refrigerators and air conditioners use instead? Several replacements are being considered.
Some groups are promoting another class of fluorine-containing compounds called hydrofluoroolefins (or HFOs). These have a short lifetime in the atmosphere and so pose much less of a climate risk. However, environmental groups have raised concern about the potentially toxic chemicals produced when HFOs break down.
Another option is to use mixtures of hydrocarbons such as butane. Hydrocarbons pose safety risks as they are highly flammable and may also adversely affect air quality. Ammonia is another alternative that has been used as a refrigerant for a long time but is highly toxic.
And, finally, there is the surprise candidate: CO₂. Although using CO₂ as a refrigerant poses technical challenges, it is non-toxic and non-flammable and a much weaker greenhouse gas than the HFCs it would replace. Strangely, from an environmental perspective, CO₂ may actually be the “best” refrigerant available.
A cooler future ahead?
The Montreal Protocol has long been considered one of the greatest environmental success stories of all time. It brought together the world’s governments and chemical industries to protect the ozone layer.
Read more: After 30 years of the Montreal Protocol, the ozone layer is gradually healing
The adoption of the Kigali Amendment will be another feather in the cap of this important agreement. HFCs aren’t overly prevalent yet – but without Kigali they are expected to grow rapidly. By banning them now, we will avoid their impacts before it is too late.
Estimates suggest that phasing out HFCs will prevent up to 0.5℃ of future warming. Even if this estimate turns out to be overly optimistic, getting rid of the HFCs will be an important step towards achieving the Paris Agreement goal of limiting warming to well below 2℃.
![]() This article was updated on November 3, to reflect that the United Kingdom have not yet ratified the Kigali Amendment.
This article was updated on November 3, to reflect that the United Kingdom have not yet ratified the Kigali Amendment.
Jenny Fisher, Senior Lecturer in Atmospheric Chemistry, University of Wollongong and Stephen Wilson, Associate Professor, University of Wollongong
This article was originally published on The Conversation. Read the original article.
UOW academics exercise academic freedom by providing expert commentary, opinion and analysis on a range of ongoing social issues and current affairs. This expert commentary reflects the views of those individual academics and does not necessarily reflect the views or policy positions of the University of Wollongong.
:format(jpg)/prod01/channel_3/assets/live-migration/www/images/content/groups/public/web/media/documents/mm/uow240113.jpg)